Relative Humidity
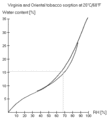 Relative Humidity (RH) is a fairy accurate way of measuring tobacco water content. To insure accuracy, use a digital hygrometer and an airtight container. Because RH measures actual/potential water vapor in the air and we want to know how moist the tobacco is, the container needs time to equalize. After opening and closing the container, the air inside the container is more like the outside air and a measurement of RH won't tell us anything. It will take an hour or so for the moisture in the air and tobacco to reach equilibrium, for the RH of the air to accurately reflect tobacco moisture content. If you've been drying the tobacco and just put it in the container its going to take a lot longer.
Relative Humidity (RH) is a fairy accurate way of measuring tobacco water content. To insure accuracy, use a digital hygrometer and an airtight container. Because RH measures actual/potential water vapor in the air and we want to know how moist the tobacco is, the container needs time to equalize. After opening and closing the container, the air inside the container is more like the outside air and a measurement of RH won't tell us anything. It will take an hour or so for the moisture in the air and tobacco to reach equilibrium, for the RH of the air to accurately reflect tobacco moisture content. If you've been drying the tobacco and just put it in the container its going to take a lot longer.
Note: Relative humidity measurements of tobacco inside an airtight container are only temporarily affected by changes temperature. Tobacco is hygroscopic, it will absorb/release water vapor from/to the air until the air has a relative humidity corresponding to the moisture content of the tobacco. Like opening and closing the container, the tobacco and air need time to equalize after changes in temperature.
Comments [ new ]
- Re: Learning curve Re: Throw it? Re: A 'Wedge
- Posted by Scott on Tuesday, 06-Nov-2007
Remember that R/H and temperature are inversely related. Decrease the temp, and R/H goes up. All that being that the container is un opened.
[ reply | link ] to this. Go to [ topic | top ]
[moved, old parent]
- Re: Learning curve Re: Throw it? Re: A 'Wedge
- Posted by Kerry on Tuesday, 06-Nov-2007
Of course, you can't stuff it without opening the package.
[ reply | link ] to this. Go to [ parent | topic | top ]
RH is a measure of the percentage of maximum moisture that the air can contain at a given temperature. The lower the temperature, the less maximum moisture the air can contain. This is why fog forms mostly when the weather is cooler. The air simply becomes saturated and can't hold the moisture which then forms water droplets or fog.
This is also why RH is a bad measure of actual moisture in the air. The cooler the air, the less actual maximum moisture it is able to contain.
Although the RH may be at 60% at 70 degrees, drop the temperature and although the same amount of moisture is available for a time, it can't be contained by the air and is essentially dropped. Dew points are a better measure of actual humidity (actual available moisture), but not perfect either.
Moisture can't escape a closed container, but in real terms, RH actually goes down in direct relation to temperature in most cases which is why many people's skin dries out during fall and winter. Of course, there are exceptions, but for the most, part cooler air means drier air, regardless of the RH.
As for the closed container, it is explained here better than I can say it:
[link]
The pressure variant isn't something that most people are willing or able to control, so it really doesn't matter for the purposes of this discussion.
This is the reason I don't recommend storing tobacco in refrigerators and especially not freezers. If you do, I would recommend removing the unopened package with enough time for it to come to "room" temperature before opening and using it. This "should" allow the tobacco to reabsorb the moisture forced out by the temperature.
Of course, this is JMHO and I could be wrong.
- Re: Learning curve Re: Throw it? Re: A 'Wedge
- Posted by mike c on Wednesday, 07-Nov-2007
I may be unable to post much for a while (though I imagine nobody believes this including myself)
[ reply | link ] to this. Go to [ parent | topic | top ]
DO NOT PUT TOBACCO IN THE FREEZER OR FRIDGE
NOT A GOOD IDEA
DON'T DO IT!!!
I'M SERIOUS!!! JUST STOP YOURSELF IF THE URGE IS STRONG
- Re: Learning curve Re: Throw it? Re: A 'Wedge
- Posted by Dave L on Monday, 12-Nov-2007
Putting tobacco in the fridge or freezer is both harmless and pointless... unless you are getting tobacco with bugs in it (given enough time, both will kill bugs). Your container should be airtight (your tobacco will stay 'fresher' at room temperature if its not because fridges and freezers have a low RH) and if using the freezer, transition to the fridge before moving to room temperature to prevent condensation.
[ reply | link ] to this. Go to [ parent | topic | top ]
- Re: Learning curve Re: Throw it? Re: A 'Wedge
- Posted by Dave L on Monday, 12-Nov-2007
Relative humidity is a measure of the amount of water vapor actually in the air compared to (my notes also say "divided by") the amount of water vapor that the air is capable of holding.
[ reply | link ] to this. Go to [ parent | topic | top ]
In an airtight container of tobacco, the only variables are the tobacco and the temperature (...air to tobacco ratio might be another). Tobacco is hygroscopic and will absorb whatever water vapor it can. It appears that, while a decrease in temperature means the air can hold less water vapor, the rise in RH is temporary because the tobacco absorbs the difference, i.e. the actual amount of water vapor in the air decreases roughly proportionate to the amount of water vapor the air is capable of holding. Its the only way I can explain a mere 1% change in RH when the temperature changed by almost 30 degrees (see my other post).
- Re: Learning curve Re: Throw it? Re: A 'Wedge
- Posted by Dave L on Monday, 12-Nov-2007
When you store tobacco in an airtight container you will see almost no change in Relative Humidity due to changes in temperature. I couldn't find my notes so I repeated the experiment with a jar 2/3 full of tobacco and a digital hygrometer.
[ reply | link ] to this. Go to [ parent | topic | top ]
66% @ 62 degrees (Sunday)
67% @ 35 degrees (Sunday night)
66% @ 65 degrees (Monday)
The hygrometer spiked high when I first put the jar in the fridge and it spiked low when I took it out. It took 3 or 4 hours for the environment inside the jar to stabilize to the readings above. I left the jar in the fridge and at room temperature (before and after) for another 8 hours to confirm that the RH numbers were solid.